
A new study has found a better way to turn a common liquid chemical into clean energy, and it could help us move away from fossil fuels.
Kate Waldie, RCEI Affiliate, an Associate Professor in the Department of Chemistry and Chemical Biology at Rutgers University, is a co-author on the study, which was published in the journal ACS Catalysis. You can read the full study here.
The authors of the study focused on formic acid, a liquid that can store energy and is safer to handle than hydrogen gas. If we can find better ways to convert formic acid into carbon dioxide (CO₂), we could use it as a cleaner fuel in fuel cells.
The team created special molecules that incorporate cobalt, a metal that is cheaper and more common than precious metals like gold or platinum. These molecules act as catalysts, meaning they help speed up the chemical reaction that turns formic acid into CO₂ and electricity. The key to their success? Tiny parts of the molecule called “pendent amines” that help the reaction happen faster and more efficiently.
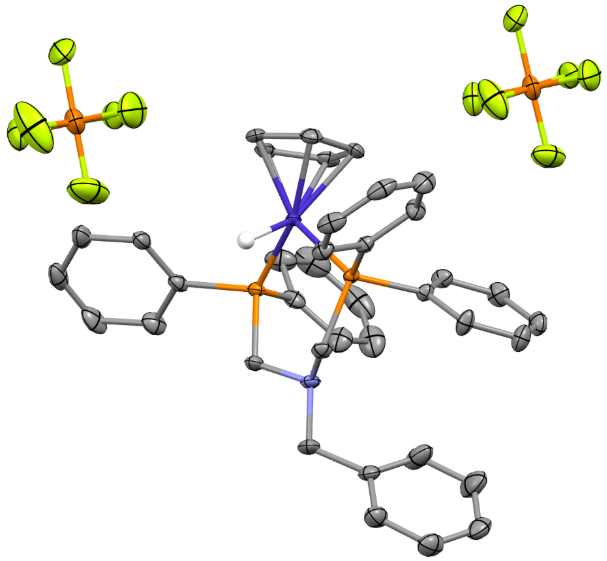
One of the cobalt catalysts worked especially well, turning formic acid into CO₂ with high efficiency at room temperature. This is important because it means the reaction produces only CO2 – no unwanted byproducts – and does not require a lot of extra energy to work.
But wait, doesn’t releasing CO₂ make climate change worse?
Great question. Normally, yes—burning fossil fuels releases CO₂ that has been trapped underground for millions of years, adding more carbon to the atmosphere. But in this case, the CO₂ comes from formic acid, which can be made from renewable sources like captured atmospheric CO₂. This creates a “closed loop” for carbon: you use CO₂ from the air to make formic acid, and then regenerate it when you need energy. No additional carbon is added to the atmosphere, making it a climate-friendly cycle.
“This research shows how we can design more sustainable catalysts to help harvest clean energy on demand,” said Kate Waldie. “By understanding how these molecules work, we’re one step closer to building better fuel systems that could power homes, vehicles, or even portable electronics in a greener way.”
Formic acid can also help us make the most of renewable electricity. Power from sources like solar and wind can be used to produce formic acid, and this stored energy can then be released in a fuel cell. Since formic acid is a liquid, it’s easier to store and transport than hydrogen gas, making it a practical option for future energy systems.
The study also highlights the importance of using earth-abundant materials like cobalt, which supports the development of a more globally competitive and sustainable energy industry.
This article was written with assistance from Artificial Intelligence, was reviewed and edited by Oliver Stringham, and was reviewed by Kate Waldie, a co-author on the study.








